Thursday, January 18, 2007
Protozoa diseases
Plasmodium, Giardia Lamblia, Crytosporidium and Entamoeba Histolytica.
Plasmodium
4 typical plasmodia that infects human includes: P. falciparum, P. vivax, P. malariae, P. ovale
Epidemiology
Generally limited to tropics and subtropics regions.
Relatively uncommon in the temperate zone, although epidemic outbreaks may occur when the largely nonimmune populations of these areas are exposed, usually unstable and relatively easy to control or eradicate.
Tropical malaria is usually more stable, difficult to control, and far harder to eradicate.
Mode of transmission
Transmission to humans occurs through the bite of female anopheles mosquitoes whereby sporozoites in mosquito saliva are injected into humans
Symptoms
-high fever
-chills
-muscle pain
-diarrhea
Clinical Findings
-Fever
Fever occurs and coincides with RBC lysis.
Periodic febrile episodes become obvious, coinciding with lysis of infected RBC.
Periodicity is 48 hours for P. falciparum, P. vivax, & P. ovale infection but 72 hours for P. malariae infection.
-Anemia
Anemia occurs as a result of RBC destruction leading to enlargement of liver and spleen.
-Large scale intravascular hemolysis
This is observed in P. falciparum infection. As a result, massive hemoglobinuria (blackwater fever) is observed due to the release of Hb from the RBC lysed intravascularly. Manifestation of intravascular hemolysis can include acute tubular necrosis and renal failure.
-Cerebral malaria
Cerebral malaria can result if P. falciparum malaria is left untreated.
Diagnosis
-Thick blood film
Microscopic examination of thick blood film stained with Giemsa’s stain at pH 7.2 helps demonstrates the presence of malarial parasites. This preparation concentrates the parasites and permits the detection even of the mild infections.
-Thin blood film
When the thick blood film demonstrates the presence of malarial parasites, further identification and confirmation of the specific malaria parasite could be done via performance of a Giemsa’s stained thin blood film that allows identification of characteristic specific to particular malaria parasites.
Chracteristics
P.falciparum has ring stage trophozoites that are small and is 1/5 of RBC diameter.
It also has 2 chromatin granules and crescentic gametocytes.
P.vivax has large rings that is ½ to 1/3 of RBC diameter and 1 chromatic granule. It has round or oval shape gametocytes.
-Serological methods
Polymerase Chain Reaction to detect Plasmodium nucleic acids or rapid diagnostic test employing the usage of dipsticks with monoclonal antibody specific against the target parasite antigen such as P. falciparum.
Prevention
-Chemoprophylaxis to travelers
-Mosquito net, window screens, protective clothing and insect repellents
-Drainage of stagnant water reduces the breeding areas
Treatment
Antimalarial grugs like Chloroquine. (1, 2)
Giardia Lamblia
Most common cause of waterborne epidemic diarrheal disease.
Common in wilderness areas because many animal carriers shed cysts into water.
Varies in severity
Mode of transmission
Parasitic in the intestines of humans and animals.
2 stages, one of which is a cyst form that can be ingested from contaminated water. Once the cyst enters the stomach, the organism is released into the gastrointestinal tract where it will adhere to the intestinal wall. Eventually the protozoa will move into the large intestine where they encyst again and are excreted in the feces and back into the environment. Once in the body, the Giardia causes giardiasis.
Symptoms
-Diarrhea
-abdominal cramps
-nausea
-weight loss
-general gastrointestinal distress.
Diagnosis
-Antigen testing of the stool. A small sample of stool is tested for the presence of Giardial proteins. The antigen test will identify more than 90% of people infected with Giardia.
-Can be diagnosed by examination of stool under the microscope; however, it takes three samples of stool to diagnose 90% of cases. Despite requiring three samples of stool, microscopical examination of stool identifies other parasites in addition to Giardia that can cause diarrheal illness. Therefore, microscopical examination of stool has value beyond diagnosing giardiasis, for example, it can diagnose other parasites as the cause of a patient’s illness.
-Collection and examination of fluid from the duodenum or biopsy of the small intestine, but these require a good deal of discomfort. The string test is a more comfortable method for obtaining a sample of duodenal fluid. For the string test, a gelatin capsule that contains a loosely-woven string is swallowed. One end of the string protrudes from the capsule and is taped to the patients outer cheek. Over several hours, the gelatin capsule dissolves in the stomach, and the string uncoils, with the last 12 inches or so passing into the duodenum. In the duodenum the string absorbs a small amount of duodenal fluid. The string then is untapped from the cheek and is removed. The collected duodenal fluid is expressed from the string and is examined under the microscope. Although more comfortable than some of the other tests, it is not clear how sensitive the string test is, for example, does it diagnose 60% (not very good) or 90% (very good) of cases of giardiasis.
Prevention
Avoiding contaminated water and the use of slow sand filters in the processing of drinking water
Treatment
Medicinally by quinacrine, metronidazole, and furazolidone. (3, 5, 6)
Cryptosporidium
Mode of transmission
Causes cryptosporidiosis. Spread by the transmission of oocysts via drinking water, which has been contaminated with infected fecal material. Oocysts from humans are infective to humans and many other mammals, and many animals act as reservoirs of oocysts, which can infect humans. Once inside of its host, the oocyst breaks, releasing four movable spores that attach to the walls of the gastrointestinal tract, and eventually form oocysts again that can be excreted.
Symptoms
-diarrhea
-headache
-abdominal cramps
-nausea
-vomiting
-low fever
Diagnosis
Polymerase Chain Reaction
Prevention
-Practice good hygiene
-Avoid water that might be contaminated
-Avoid food that might be contaminated.
Treatment
No treatment against the protozoa, patients will usually recover, but the disease can be fatal in late stage AIDS patients. (4, 5, 6)
Entamoeba histolytica
Mode of transmission
Via contaminated food and water
It is another water-borne pathogen that can cause diarrhea or a more serious invasive liver abscess. Ingested cysts excyst in the intestine and proteolytically destroy the epithelial lining of the large intestine.
Clinical Findings
May be asymptomatic to fulminating dysentery, exhaustive diarrhea, and abscesses of the liver, lungs, and brain.
Treatment
Several antibiotics
Prevention
Avoiding contaminated water, hyperchlorination or iodination can destroy waterborne cysts. (5, 6)
1. Brooks, G.F., Butel, J.S. & Ornston, L.N. (2004). "Jawetz, Melnick & Adeberg's Medical Microbiology", 23rd edition, Appleton & Lange.
2. http://health.yahoo.com/eney/healthwise/hw119119
3. http://www.medicinenet.com/giardia_lamblia/page3.htm
4. http://en.wikipedia.org/wiki/cryptosporidium
5. http://pages.cabrini.edu/sfuller-espie/Microbiology%20Lecture20Outlines/micro_fungal_protozoan_diseases.htm
6. http://udel.edu/~dlehman/bisc300/fungi.html
Suspected Fungi
Dermatophytes
Dermatophytes, or also known as keratinophilic fungi, produce extracellular enzymes (keratinases) which are capable of hydrolyzing keratin.
There are 3 genera of dermatophytes:
1. Trichophyton species (19 species)
- These infect skin, hair and nails. Rarely can cause subcutaneous infections, in immunocompromised individuals.
- Can be identified by their colony appearence and microscopic morphology.
- Grow for 2 weeks at 25 degree C on Sabouraud's dextrose agar.
- Colonies of T. mentagrophyts may be cottony to granular, display abundant grape-like clusters of spherical micoconidia(small reproductive structures)


 http://www2.provlab.ab.ca/bugs/webbug/mycology/tment.htm
http://www2.provlab.ab.ca/bugs/webbug/mycology/tment.htm
 http://www2.provlab.ab.ca/bugs/webbug/mycology/trub.htm
http://www2.provlab.ab.ca/bugs/webbug/mycology/trub.htm http://www2.provlab.ab.ca/bugs/webbug/mycology/ttons.htm
http://www2.provlab.ab.ca/bugs/webbug/mycology/ttons.htm2. Microsporum species (13 species).
- These may infect skin and hair, rarely nails.
- Produce distinctive multicellular macroconidia with echinulate walls.

-M canis forms a colony with a white cottony surface and deep yellow on the reverse. 8 to 15-celled macroconidia frequently have hooked tips.

 http://www2.provlab.ab.ca/bugs/webbug/mycology/mcanis.htm
http://www2.provlab.ab.ca/bugs/webbug/mycology/mcanis.htm http://www2.provlab.ab.ca/bugs/webbug/mycology/mgyp.htm
http://www2.provlab.ab.ca/bugs/webbug/mycology/mgyp.htm1. It is usually found in the soil and trasmitted to man by direct exposure.
2. It is transmitted to man through close contact with animals (cats, dogs, cows) or with contaminated products.
3. It can be transmited anthrophphilic, which is transmission from man to man by close contact ot through contaminated objects. [1]
Tinea unguium (onychomycosis) - nails. Clipped and used for culture
Tinea capitis - head. Frequently found in children.
Tinea barbae - ringworm of the bearded areas of the face and neck
Diagnosis is usually possible by direct microscopic examination of KOH-treated skin scrapings which show a typical aspect of mycelia and spores. [1]
Treatment:
-Ketoconazole
-Itraconazole - oral
-Terbinifine (Lamisil) - oral, topical. [1]
Saturday, December 16, 2006
Compilation for Patients Diagnosed with UTI
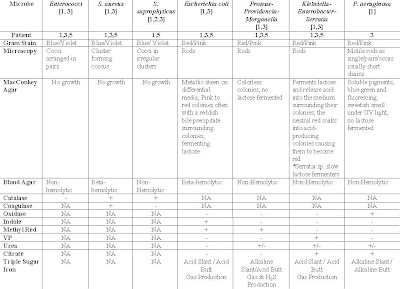 Table 1: Suspected Organisms, Relevant tests and expected results of tests for respective patients diagnose with UTI
Table 1: Suspected Organisms, Relevant tests and expected results of tests for respective patients diagnose with UTI2) Kenneth Todar. (2004). Staphylococcus. On site http://www.textbookofbacteriology.net > Google Search > Retrieved on 5th December 2006.
Thursday, December 14, 2006
Thursday, December 7, 2006
MAYBELLINE WRITES!
Age: 26 Years
Sex: Female
Clinical Diagnosis
Complaints: Fever, chills, dysuria (pain during urination)
Diagnosis: Urinary Tract Infection
Antibiotic treatment: Nil
Urinary Tract Infection (UTI)
UTI is an infection that can happen anywhere along the urinary tract. It is usually caused by a bacterium from the anus entering the urethra and then the bladder. UTI is more common in women because their urethra is shorter and closer to the anus. (1) It may also be caused by dehydration, obstruction, disturbance of smooth urinary flow or presence of a foreign body, e.g. stone or urinary catheter. Trauma during sexual intercourse may also cause and infection in women. (2)
Usually, an UTI infected patient will experience dysuria, hematuria (bloody urine), fever, cystitis ( pain in the midline suprapubic region and/or frequent urination) and urethritis (discomfort or pain at the urethral or a burning sensation) .
The more common and suspected microorganisms that cause UTI are Escherichia coli (E.coli), Klebsiella pneumoniae, Proteus mirabilis, Psedonmonas aeruginosa. Staphylococcus saprophyticus and Enterococcus spp.
Gram-negative E.coli, Klebsiella and Proteus
10-20% of sexually active, young women are infected by Staphylococcus saprophyticus causing UTI. Staph. saprophyticus are gram-positive, cocci in irregular clusters and are facultative anaerobes. They are coagulase-negative and catalse-postive. (5, 6)
Entercococcus spp. are classified under Group D of the heterogeneous group of the Streptococci family. Streptococci are gram-positive spherical bacteria that form pairs or chains during growth. Group D Entercocci are usually non-hemolytics and occasionally alpha-hemolytic and are PYR positive. (7)
Diagnostics Laboratory Tests
The diagnosis of UTI can be confirmed by a urine culture. The urine culture will be sub cultured, isolated to identify the bacteria by running a series of appropriate biochemical tests. An antibiotic susceptibility testing will also be done to see which antibiotic is most suitable to be used to treat the patient. In diagnosis of UTI, if the urine culture yielding a greater than 100,000 colony-forming units (CFU)/mL and pure growth would indicate a significant bacteria growth. Or a urine culture that yield >100,000 CFU/mL with 2 bacteria growth may also indicate significant growth. However, if more than 3 organism is present, it indicates mixed bacteria growth and may be due to bad sample collection or contamination.
The urine culture is sub-cultured into TSA with 5% sheep blood agar (blood agar) and Cysteine Lactose Electrolyte (CLED agar).
The agar plates are left to incubate in the incubator at 37o C for 18-24hrs. The media is streak in a systematic manner as shown below.

http://uths.revealed.net/science/stein/homework/lab_ident_bacteria_intro.htm
GRAM POSITIVE COCI

www.bmb.leeds.ac.uk/.../barbercase.html
Gram-positive cocci in clusters may indicate Staphylococcus saprophyticus while gram-positive cocci in pairs or chains may indicate Enterococcus spp..
If the gram-stain showed a gram-positive cocci, the following tests can be done.

Catalase
With an inoculating loop, pick a small colony and swab it onto a glass slide. Then, add 2-3 drops of hydrogen peroxide to the colony. A catalase positive test will show bubbles form immediately.
The catalase test differentiates the Staphylococci, which are positive, from the Streptococci, which are negative. When a catalase test is positive, it will produce bubbles. This is because Staphylococci produce catalase that will convert hydrogen peroxide to water and oxygen, producing bubbles.
(+) (-)

http://faculty.mc3.edu/jearl/ML/ml-10.htm
Coagulase
With an inoculating loop, pick a small colony and swab it onto a glass slide. Then, add 2-3 drops of coagulase to the colony. Mix the coagulase with the colony well and observe for agglutination.
When a catalase is positive, proceed to do a coagulase test to differentiate S.aureus from other Staphylococci. S.aureus produce coagulase, an enzyme-like protein that clots citrated plasma. Coagulase will bind to prothrombin and initiate fibrin polymerization. Coagulase may deposit fibrin on the surface of Staphylococci. When this happen, S. aureus will produce a clumping factor on its surface for the organism’s adherence to fibrinogen and fibrin. Agglutination indicates coagulase positive, indicating that the organism is most likely a S. aureus. Coagulase negative suggest it might be other species of staphylococci. In the case of UTI, it might be a S. saprophyticus.
(-) (+)

http://faculty.mc3.edu/jearl/ML/ml-10.htm
When a catalase test is negative, observe the blood agar and check for hemolysis. Blood agar is an enriched, differential media used to isolate fastidious organisms and detect hemolytic activity. β-hemolytic activity will show complete lysis of red blood cells surrounding colony, while α-hemolysis will only partially lyse hemoglobin and will appear green. non-hemolysis or γ-hemolysis is the term referring to a lack of hemolytic activity.
When a catalase test is negative, observe the blood agar and check for hemolysis. Blood agar is an enriched, differential media used to isolate fastidious organisms and detect hemolytic activity. β-hemolytic activity will show complete lysis of red blood cells surrounding colony, while α-hemolysis will only partially lyse hemoglobin and will appear green. non-hemolysis or γ-hemolysis is the term referring to a lack of hemolytic activity. jade.ccccd.edu/mweis/.../media_SD_list_page.htm
jade.ccccd.edu/mweis/.../media_SD_list_page.htmα-hemolysis
 jade.ccccd.edu/mweis/.../media_SD_list_page.htm
jade.ccccd.edu/mweis/.../media_SD_list_page.htm
γ-hemolysis
 www.sph.unc.edu/courses/eric/dd_cs/betahemo.htm
www.sph.unc.edu/courses/eric/dd_cs/betahemo.htm
For α-hemolytic results, an Optochin test can be done. Optochin is an antibiotic susceptibility testing. This test is to differentiate between α-hemolytic Streptococci which is resistant to Optochin and S. pneumoniae which is sensitive to Optochin.
If the organism is sensitive to Optochin, it will produce a clear zone surrounding the colony.
For γ -hemolytic results, a Pyrulidonyl Peptidase (PYRase activity) test can be done. PYR is a rapid colourmetric test for use in the differentiation of Enterococci form Lancefield Group D Streptococci. PYR disc is impregnated with PYR, a substrate that is hydrolyzed by pyrase to form β-naphthylamine. They hydrolase forms a red compound upon addition of a colour developer. Pyrase activity is present in Entercocci. If pink or orange-pink colour is formed, it indicates a positive PYR test, showing presence of the organism Enterecocci. If no colour is form, it shows a negative PYR test, showing presence of non-hemolytic Streptococci.
 Oxidase
OxidaseOxidase test is done to determine the presence of cytochrome oxidase activity in bacteria. Cytochrome oxidase is an enzyme found in certain bacteria that is able to transfer electrons to oxygen. The enzyme oxidizes reduced cytochrome c to make this transfer of energy. Presence of cytochrome oxidase can be detected through the use of oxidase disk which acts as an electron donator to cytochrome oxidase. If the bacteria oxidize the disk, the disk will turn purple, indicating a positive test. No colour change indicates a negative test. (8)
 http://medic.med.uth.tmc.edu/path/oxidase.htm
http://medic.med.uth.tmc.edu/path/oxidase.htm
If oxidase is positive, do a sensitivity testing on Mueller Hinton agar. P. aeruginosa will produce green pigment colonies.

If oxidase test is negative, observe the colonies form on CLED agar. CLED agar is used to differentiate between lactose fermenters and non-lactose fermenters bacteria. It also prevents the swarming of Proteus species. Lactose fermeters produce yellow colonies on CLED agar while non-lactose fermenters appear blue.

www.wign.sk/imuna/sk/produkt.php?c=43&b=2
For lactose fermenter bacteria, proceed to do an Indole test. Indole is one of the degradation products of amino acid metabolism. It is useful in identifying E. coli. The test is based on the formation of a red complex when indole reacts with the aldehyde group of an active chemical in kovac reagent. A positive test will produces pink/purple colour at interface and the organism is E. coli. A negative test will remain colourless and further testing can be done to confirm if it is Enterococci or Klebsiella.
References:
1. Wikepidia (2006). On-site: http://en.wikipedia.org > search >. Retrieved on 4th December 2006.
2. Medical Microbiology and Infection at a Glance Pg 94
3. Geo FB, Janet SB & Stephen AM. (2004). Jawetz, Melnick, & Adelberg’s Medical Microbiology. 23rd edition. McGraw-Hill.Pg 248
4. Geo FB, Janet SB & Stephen AM. (2004). Jawetz, Melnick, & Adelberg’s Medical Microbiology. 23rd edition. McGraw-Hill. Pg 262
5. On-site: year2>mmid>bms5300>bugs>stasapro">http://medinfo.ufl.edu>year2>mmid>bms5300>bugs>stasapro
6. Geo FB, Janet SB & Stephen AM. (2004). Jawetz, Melnick, & Adelberg’s Medical Microbiology. 23rd edition. McGraw-Hill. Pg 223
7. Geo FB, Janet SB & Stephen AM. (2004). Jawetz, Melnick, & Adelberg’s Medical Microbiology. 23rd edition. McGraw-Hill.Pg 231, 235
8. On-site: intranet-web>Courses>DMI_8351>Oxidase">http://dentistry.ouhsc.edu>intranet-web>Courses>DMI_8351>Oxidase
Patient 2 - Posted by Hui Yan.
Age: 28 Gender: Female
Complaints: Diarrhoea
Diagnosis: Enterocolitis
Antibiotic treatment: Nil
Stool specimen is collected from the patient.
Possible Organisms that causes enterocolitis
1. Salmonella sp.
It is a type of enterobacteriaceae. This type of organism causes bloody diarrhea with mucus.
Key Characteristics of Salmonella sp.
- Gram-negative, motile rods
- facultative anaerobes
- Non-lactose fermenters
- Produces H2S
This organism causes Salmonella enterocolitis. Salmonella enterocolitis is an infection in the lining of the small intestine. Salmonella enterocolitis can range from mild to severe diarrheal illness. The infection is acquired through ingestion of contaminated food or water. Any food can become contaminated during preparation if conditions and equipment for food preparation are unsanitary.
2. Shigella sp.
It is a type of enterobacteriaceae. This type of organism causes watery diarrhea in later stage of disease, the stool contains blood, mucus or pus. The most common symptoms are diarrhea, fever, nausea, vomiting, stomach cramps, and straining to have a bowel movement.
Key Characteristics of Shigella sp.
- Gram-negative, non-motile and non-spore forming rods
- Does not produce H2S
- Aerobic microbe
This organism cause Shigellosis. Shigellosis or known as Shigella enterocolitis is a common cause of acute diarrhea in adults.
3. Campylobacter jejuni
It is gram-negative, curved, rod-shaped bacteria. It is motile with a single polar flagellum and it is micro-aerophillic type of microorganism.
Infection with C. jejuni usually results in enteritis (inflammation of small intestines), which is characterised by abdominal pain, diarrhea, fever, and malaise. Diarrhea can vary in severity from loose stools (watery stools) to bloody stools.
4. Entameba histolytica
Entamoeba histolytica is an anaerobic parasitic eukaryote protozoan. It infects predominantly humans and other primates. The active (trophozoite) stage exists only in the host and in fresh feces; cysts survive outside the host in water and soils and on foods, especially under moist conditions on the latter. When swallowed they cause infections by excysting (to the trophozoite stage) in the digestive tract.
5. Clostridial organisms- Clostridium difficile
Clostridial difficile is a species of bacteria of the genus Clostridium which are gram-positive, anaerobic, spore-forming rods. C. difficile is the most significant cause of pseudomembranous colitis, a severe infection of the colon, often after normal gut flora is eradicated by the use of antibiotics.
Since the patient is an outpatient and she does not have any antibiotic treatment, thus Clostridium difficile is not the cause of enterocolitis as it often is caused by antibiotic treatment and commonly, this type of infection is acquired in the hospital.
6. Giardia lamblia
Giardia lamblia (formerly also Lamblia intestinalis and also known as Giardia duodenalis and Giardia intestinalis) is a flagellated protozoan parasite that infects the gastrointestinal tract and causes giardiasis. Infection causes giardiasis, a type of gastroenteritis that manifests itself with severe diarrhea and abdominal cramps. Other symptoms can include bloating, flatulence, fatigue, nausea, vomiting and weight loss. Giardia is a major cause of intestinal disease worldwide.
7. Enteropathogenic E. Coli (EPEC)
EPEC is a gram-negative bacillus (rod-shaped organism). EPEC causes a profuse watery diarrheal disease and it is a leading cause of diarrhea in developing countries for infants.
The patient is 28 years old, thus EPEC is not the cause of enterocolitis.
- Salmonella sp.
- Shigella sp.
- Campylobacter jejuni
- Entameba Histolytica
- Giardia lamblia
Investigational tests
1. Microscopy test
A. Gram staining
This test is to find out whether the microorganism is gram positive or gram negative and the shape of the organism (coccus or bacillus). After that, relevant biochemical tests can be done to find out the identity of the suspected organisms.
B. Wet mount
This is used for checking for the presence of pus, blood and any parasites in the stool sample and motility of the microbes.
C. Stool ova and cyst
This test is to check for the presence of cyst and/or ova in the stool.
D. Parasite
This test is to check whether there is any presence of parasite in the stool.
2. Culture (Fecal) - allow the microbes to become enriched in numbers – e.g. using peptone and selenite broth.
3. Serology tests such as slide agglutination tests and Widal tests (tube agglutination) – this is to test whether the suspected microorganism reacts to certain antigens such as O, K, H and Vi antigens.
4. Kirby-Bauer test (using antibiotics discs) or known as Antibiotics Susceptibility test – this is to test whether the cause of enterocolitis is caused by the abnormal flora of certain microorganisms that are resistant to antibiotics
5. Other possible tests
A. Using Salmonella-Shigella agar – to find out whether the suspected microorganism is either Salmonella or Shigella sp.
B. Triple-Sugar Iron test – to find out whether the suspected microorganism ferments any of the 3 sugars (lactose, glucose, fructose) and whether it produce gas or not.
C. Using Campylobacter selective media at 42oC, 10% carbon dioxide, 3-4 days incubation – this is to find out whether the Campylobacter sp. is the cause of enterocolitis. This selective media only allows Campylobacter sp. to grow.
D. MacConkey agar – a selective media to grow Salmonella strains.
It is a selective and differential media used to differentiate between Gram negative bacteria while inhibiting the growth of Gram positive bacteria. The addition of bile salts and crystal violet to the agar inhibits the growth of most Gram positive bacteria, making MacConkey agar selective.
E. Xylose lysine deosycholate (XLD)agar – a selective growth media used in the isolation of Salmonella and Shigella species from clinical samples.
XLD also can be used for the culture of stool samples, and contains two indicators. It is formulated to inhibit Gram-positive bacteria, while the growth of Gram-negative bacilli is encouraged. The colonies of lactose fermenters appear yellow.
F. Blood Agar plate (BAP) - Contains mammalian blood (usually sheep), typically at a concentration of 5–10%. BAP are an enriched, differential media used to isolate fastidious organisms and detect hemolytic activity.
G. Hektoen Enteric (HE) - HE agar is designed to isolate and recover fecal bacteria belonging to the Enterobacteriaceae family. HE is particularly useful in isolating Salmonella and Shigella.
Reference
Brooks, G. F., Butel, J. S. & Ornston, L. N.; “Jawetz, Melnick & Adeberg’s Medical Microbiology”, 23rd edition, Appleton & Lange, 2004.
http://www.nlm.nih.gov >medlineplus >salmonella enterocolitis
http://www.wikipedia.org >agar_plates
http://www.wikipedia.org > Campylobacter jejuni
http://www.wikipedia.org >Entameba Histolytica
http://www.wikipedia.org >Giardia lamblia
http://www.wrongdiagnosis.com >enterocolitis
Patient 3 - Maisy Wong
Maisy Wong,
Female, 66 years old
*Note: It is also known that the patient is an in-patient of the hospital based on the ward and bed number of the patient provided.
Clinical Diagnosis
Complaints: Fever, chills, bladder distension (stretching); on indwelling catheter
Diagnosis: Urinary Tract Infection (UTI)
Antibiotic Treatment (if any): Nil
*Note: A catheter is a hollow tube that is used to drain urine from the bladder. An indwelling catheter stays in place for long periods of time (Long time catheter). There are two kinds of indwelling catheters: urethral and supra pubic. A urethral catheter is inserted into the bladder through the urethra. A supra pubic catheter is inserted into the bladder through a hole in the abdomen, a few inches below the tummy button[8].
Specimen: Urine
Collection of specimen: As an indwelling catheter is in place, the urine should be obtained by sterile aspiration of the catheter with needle and syringe but not from the collection bag. To resolve diagnostic problems, urine can be aspirated aseptically directly from the full bladder by means of suprapubic puncture of the abdominal wall[2].
Suspected Organisms:
(i) Streptococci species (Entercocci)
(ii) Enterobacteriaceae (E. coli, Proteus-Providencia-Morganella, Klebsiella-Enterobacter-Serratia)
(iii)Gram Neg Bacilli (Pseudomonas aeruginosa, Acinetobacter species)
Key Characteristics of respective organisms [2,6]:
(A)Enterococci
· Gram positive cocci arranged in pairs
· Facultative anaerobes2
· Do not contain catalase enzyme, thus, catalase negative
· Non-hemolytic aka gamma hemolytic
· Bile-esculin positive
· Able to grow in 6.5% NaCl
B) Enterobacteriaceae species
· Gram negative rods
· Facultative anaerobes
· Catalase positive
· Oxidase negative
(Bi)E. coli
· Member of normal intestinal flora
· Rapidly ferment lactose
· Beta-hemolytic
· Produce positive indole test
· Positive for b-glucoronidase using the substrate (MUG)
· Ferments mannitol
(Bii)Proteus-Providencia-Morganella
· Does not ferment lactose
· Motile
· Grow on potassium cyanide medium
· Ferment xylose
· “Swarming” on solid media
· Urease positive for Proteus species and Morganella morganii
· Urease negative for Providencia species
(Biii)Klebsiella-Enterobacter-Serratia
Klebsiella species
· Exhibit mucoid growth
· Lacks motility
· Lysine Carbohydrate positive
· Citrate positive
· Have large polysaccharide
· Voges-Proskauer positive
· Rapidly ferment lactose
Enterobacter species
· Motile
· Citrate positive
· Ornithine decarboxylase positive
· Produce gas from glucose
· Voges-Proskauer positive
· Rapidly ferment lactose
Serratia
· Produces DNase, lipase and gelatinase
· Voges-Proskauer positive
· Slow fermenter of lactose
(C) Pseudomonas species
(Ci)P. aeruginosa
· Gram negative motile rods as single/pairs/occasionally short chains
·Grows well at 37-42 degree Celsius (Ability to grow at 42 degree celsius helps in differentiation)
· Oxidase positive
· Able to produce water soluble pigments such as pyocyanin(bluish & nonfluorescent), pyoverdin(greenish & fluorescent) & pyorubin (red-brown).
· Does not ferment lactose
(Cii)Acinebacter species
· Short
· Plump coccobacilli
· Oxidase negative
(A) Culture Medias
· MacConkey agar
MacConkey agar is a culture medium designed to grow Gram-negative bacteria and stain them for lactose fermentation. It contains bile salts, crystal violet dye (to inhibit Gram-positive bacteria), neutral red dye (which stains microbes fermenting lactose), lactose and peptone. By utilizing the lactose available in the medium, Lac+ bacteria such as Escherichia coli will produce acid, which lowers the pH of the agar below 6.8 and results in the appearance of red colonies. Lac- bacteria that cannot utilize lactose will use peptone instead. This forms ammonia, which raises the pH of the agar, and leads to the formation of white colonies[6]. Thus, usage of this agar allows a rapid, presumptive identification of gram negative enteric bacteria[2].
· Blood Agar Plate
Blood Agar Plate contains mammalian blood (usually sheep), typically at a concentration of 5–10%. BAP are an enriched, differential media used to isolate fastidious organisms and detect hemolytic activity[6]. β-hemolytic activity will show complete lysis of red blood cells surrounding colony, while α-hemolysis will only partially lyse hemoglobin and will appear green. γ-hemolysis is the term referring to a lack of hemolytic activity[6]. Most aerobic and facultative anaerobes will grow in blood agar[2].
*Note: On receipt of the urine sample(s), there is a need to utilize the sample and culture them on culture media as soon as possible. The reason being that many types of microorganisms multiply rapidly in urine at room or body temperature and the multiplication of the organisms (contaminants), could interfere with accurate interpretation of the results.
Other then the pathogenic microorganism, Contaminating microorganisms such as the normal flora in the urethra might grow in the cultures as well even when a full voided urine is used. Nevertheless, these microorganisms could be identify as they are usually present at values lower than 10x2 to 10x4 per mL[2].
(B) Gram Staining and Microscopy
Performed using colonies that are cultured above (Gram positive stained blue/violet, Gram Negative is stained red/pink)[2].
(C) Biochemical Tests
When Gram Negative Bacilli is observed, the suspected microbe could be further tested with the oxidase test. Usage of the oxidase test helps to differentiate between the Enterobacteriacease species (Oxidase negative) and Pseudomonas species (Oxidase positive). Further identification of the specific microbe will involve:
1. Oxidase negative: Performance of Triple sugar iron sugar, Indole, Methyl Red, Citrate, Urea and Voges-Proskauer tests. Results of respective microbe is presented in Table 1
2. Oxidase positive: Performance of API 20 NE, Pseudomonas Agar F and Pseudomonas Agar P
Note: Pseudomonas Agar P favours the formation of pyocyanin and/or pyorubin and reduces that of fluorescein, whereas Pseudomonas Agar F stimulates the production of fluorescein and reduces that of pyocyanin and/or pyorubin. Simultaneous use of both culture media allows rapid, preliminary identification of most Pseudomonas species, as some strains can only synthesize pyocyanin, some form only fluorescein and others produce both pigments[1].
When Gram Positive Cocci is observed, the suspected microbe could be further tested with the catalase test. A negative catalase test is an indication for the possibility of the presence of the organisms from the Streptococcus species. Further identification of the specific microbe may involve:
Bile Esculin Agar
The bile esculin agar is a selective and differential agar. Bile salts are the selective ingredient while the esculin is the differential component. Enterococci are able to grow in the presence of 4% bile and hydrolyze the esculin to products that react with ferric citrate in the medium to produce insoluble iron salts resulting in the blackening of the medium[6].

Capsular swelling tests
specific antibody and the target antigen for the antibody. Latex agglutination assays are available for the rapid presumptive identification of several bacterial pathogens of humans and are easily performed in a physician's office. The speed of the test allows the physician to initiate therapy immediately; changes in therapy might need to be made later based on a more thorough series of diagnostic tests on the patient's isolate and on findings from antibiotic susceptibility testing for the isolate[3].
In this test, the group-specific antibodies are coated onto polystyrene latex beads. When the latex beads are incubated with an extract containing the released corresponding group-specific carbohydrate antigen, a strong antigen-antibody reaction occurs, that crosslinks the beads in a clearly observable agglutination reaction[3].
(E) Disk Diffusion Susceptibility Testing
The disk diffusion test measures the ability of drugs to inhibit the growth of the bacteria by determining the minimum diameter of inhibition zone for each respective drug. The results correlate reasonably well with therapeutic response in those disease processes where body defenses can frequently eliminate infectious microorganisms[2].
Enterococci:Vancomycin is the drug of choice. Ampicillin and nitrofurantonin is used to treat patients in umcomplicated UTI.
1. EMD Chemicals Inc. Pseudomonas Agar F Base. On site: http://www.emdchemicals.com> Search. Retrieved on 6th December 2006.
2. Geo FB, Janet SB & Stephen AM. (2004). Jawetz, Melnick, & Adelberg’s Medical Microbiology. 23rd edition. McGraw-Hill.
3. Jacob RJ & Thomson JM. (2000). Culture Media and Biochemical Tests. On site: http://www.mc.uky.edu Retrieved on 5th December 2006.
4. UMDNJ-School of Osteopathic Medicine. (2004). On-site: http://www3.umdnj.edu> case2gramnegatives. Retrieved on 7th December 2006.
5. UMR Microbiology. (2004). On-site: http://web.umr.edu/ > S_marcescens. Retrieved on 7th December 2006.
6. Wikipedia. (2006). On-site: http://en.wikipedia.org > search >. Retrieved on 4th December 2006.
7. IID Laboratory Manual. (2002). On-site: http://medteach.mccs.uky.edu/COM/iid98/manual > Lab 06. Retrieved on 7th December 2006.
8. In contact. (2006).
http://www.incontact.org/ > search > indwelling catheter. Retrieved on 4th December 2006.

